Day 2 :
Keynote Forum
Michael R Migden
University of Texas MD Anderson Cancer Center, USA
Keynote: Investigator-assessed efficacy and safety of sonidegib 200 mg QD in patients with locally advanced basal cell carcinoma: Results of the BOLT 30-month analysis
Time : 09:00 to 09:30

Biography:
Michael Migden is a distinguished US-based Dermatologist and an Associate Professor, Departments of Dermatology, Division of Internal Medicine, and Head and Neck Surgery, Division of Surgery, at the University of Texas MD Anderson Cancer Center, Houston, TX, USA. At MD Anderson, he is a Program Director of the ACGME Fellowship: Micrographic Surgery and Dermatologic Oncology. He also serves as a Faculty Member in the Department of Ophthalmic Plastic and Reconstructive Surgery. He has served as a Principal Investigator for studies on the smoothened inhibitors sonidegib, vismodegib, and taladegib, and on immune therapy trials in non-melanoma skin cancer. He has published numerous primary and expert review articles on basal cell carcinoma.
Abstract:
The 200-mg dose of sonidegib, a selective smoothened inhibitor that blocks hedgehog pathway signaling, was approved in Europe and the United States for the treatment of patients with locally advanced basal cell carcinoma (laBCC) who are not amenable to curative surgery/radiation. Approvals were based on results from the pivotal BOLT study (NCT01327053). Investigator-assessed efficacy and safety data of sonidegib 200 mg QD from the 30-month analysis are reported here.
Methodology & Theoretical Questions: Patients with laBCC were randomized to receive sonidegib 200 or 800 mg daily; here we discuss the 200-mg dose. Investigators evaluated objective response rate (ORR); complete response [CR]+partial response [PR]), duration of response (DOR), and progression-free survival (PFS) per modified response evaluation criteria in solid tumors (mRECIST; laBCC) and RECIST v1.1 (mBCC); overall survival (OS) was also assessed.
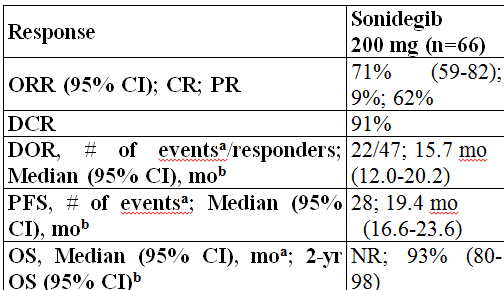
Findings: In patients with laBCC who received sonidegib 200 mg (n=66), the investigator-assessed ORR was 71%. Disease control rate was 91%; median DOR was 15.7 months, with 25/47 responders maintaining an objective response. The median PFS was 19.4 months and the median OS was not yet reached. One death was reported; it was not considered related to study treatment by investigators. The safety profile of sonidegib 200 mg was manageable; however, grade 3/4 adverse events (AEs; 43%), AEs requiring dose interruptions/reductions (43%), and/or discontinuations due to AEs (30%) were reported. Commonly reported AEs included muscle spasms (54%), alopecia (58%), and dysgeusia (60%).
Conclusion & Significance: In the BOLT 30-month analysis, sonidegib 200 mg QD provided sustained efficacy and long-term safety in patients with laBCC. Notably, these data were investigator-assessed, which are typically higher than data that are centrally reviewed. These data support the use of sonidegib 200 mg in difficult-to-treat patients with laBCC according to local treatment guidelines.
Recent Publications
1. Chen L, Silapunt S, Migden M R (2016) sonidegib for the treatment of advanced basal cell carcinoma: A comprehensive review of sonidegib and the BOLT trial with 12-month update. Future Oncol. 12(18): 2095-105.
2. Dummer R, Guminski A, Migden M R (2016) The 12-month analysis from Basal cell carcinoma outcomes with LDE225 treatment (BOLT): A phase II, randomized, double-blind study of sonidegib in patients with advanced basal cell carcinoma. J. Am. Acad. Dermatol. 75(1): 113-125.
3. Migden M R, Guminski A, Gutzmer R (2015) Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): A multicentre, randomized, double-blind phase 2 trial. Lancet Oncol. 16(6): 716-28.
Keynote Forum
Anu T Singh
Dabur Research Foundation, India
Keynote: Development and preclinical evaluation of a novel polyherbal product for treatment of Atopic dermatitis & other chronic dermal inflammatory diseases by multiparametric analysis
Time : 09:30 to 10:00

Biography:
Anu T Singh is the Vice President (R&D) at Dabur Research Foundation, India. She holds a PhD degree in Tumor Biology from All India Institute of Medical Science, Delhi and did her Post-doctoral research in Cell Signaling from National Institute of Immunology, Delhi. She has published and presented more than 60 research papers in peer reviewed journals and scientific meetings. She has extensive experience in the area of oncology, inflammation and dermapathology.
Abstract:
Eczema or atopic dermatitis (AD) is a common chronic or recurrent inflammatory pruritic skin disease with impaired skin barrier with itchy, red, swollen, and cracked skin. Skin lesions are characterized by infiltrating lymphocytes, monocytes/macrophages, eosinophils and over secretion of inflammatory cytokines/chemokines. Thymus and activation regulated chemokine (TARC) is overexpressed in eczema lesions and attracts Th2 cells. IgE and IgE-mediated mast cell, and eosinophil activation contribute to severity of eczema. Current management strategies include oral medications, steroid creams and light therapy. There is an unmet need for development of herbal based therapeutic agents with anti-AD potential. We have developed a novel aqueous mixture (SIRB-001) consisting of 3 traditional Chinese medicine (TCM) based herbs; Rheum palmatum L. (Da Huang), Rehmannia glutinosa Libosch (Sheng di huang) and Lonicera Japonica (Jin yin hua) in the ratio 1:1:3. SIRB-001 has previously demonstrated promising anti-psoriatic activity in pre-clinical and clinical studies. SIRB-001 led to inhibition of hyper-proliferation, induction of apoptosis in keratinocytes, inhibition of cytokines in keratinocytes and immune cells. SIRB-001 cream was developed and potential clinical efficacy was observed in psoriasis and scalp psoriasis. SIRB-001 was further tested for efficacy in eczema and AD using pre-clinical models. SIRB-001 demonstrated significant inhibitory effects on secretion of inflammatory cytokines, TNF-α, IFN-g, IL-6 and chemokine TARC in keratinocytes (HaCaT). Vascular endothelial growth factor (VEGF) causes hyperpermeability of blood vessels and endothelial cell proliferation, leading to persisting erythema and edema in AD. Inhibition of VEGF secretion by HaCaT indicates anti-angiogenic role of SIRB-001. Downregulation of IL-6 in RAW264.7 cells and IgE in human myeloma cell line-U-266 were observed. Janus-Kinase (JAK) is involved in cell signaling pathways activated by cytokines. SIRB-001 exhibited an inhibition of JAK-1/JAK-3 in vitro. In conclusion, based on the observed mechanistic action, SIRB-001 may be a highly effective new treatment for management of atopic dermatitis and eczema.
Keynote Forum
Manu Jaggi
Dabur Research Foundation, India
Keynote: Clinical development of a novel polyherbal product for treatment of atopic dermatitis and other chronic dermal inflammatory diseases
Time : 10:00 to 10:30

Biography:
Manu Jaggi holds a Doctorate in Cancer Biology from National Institute of Immunology, Delhi and Post-graduate in Pharmaceutical Sciences. He is the Chief Scientific Officer of Dabur Research Foundation (DRF). He has extensive experience in the area of skin-care biology and screening of variety of cosmeceutical agents. He holds more than 100 patents and has published and presented more than 150 research papers in peer reviewed journals and scientific meetings. A comprehensive range of screening assays for studying the skin-health and anti-aging potential has been developed.
Abstract:
Atopic dermatitis (AD), also known as atopic eczema, is a type of inflammation of the skin (dermatitis). It results in itchy, red, swollen, and cracked skin. Current management strategies include oral medications, steroid creams and light therapy. We have developed a novel aqueous mixture (SIRB-001) consisting of three traditional Chinese medicine (TCM) based herbs; Rheum palmatum L. (Da Huang), Rehmannia glutinosa Libosch (Sheng di huang) and Lonicera Japonica (Jin yin hua) in the ratio 1:1:3. SIRB-001 based cream was developed and found to be highly safe in animal studies. SIRB-001 has previously demonstrated promising anti-psoriatic activity in pre-clinical models and clinical efficacy in psoriasis and scalp psoriasis. SIRB-001 exhibited anti-eczema properties in cell based models. Inhibition of cytokines and IgE was observed in keratinocytes (HaCaT)/immune cells and myeloma cell line-U-266, respectively. Inhibition of JAK-1/JAK-3 was induced by SIRB-001. Encouraging preclinical results paved the path for clinical investigations in atopic dermatitis. The efficacy, safety and tolerance of SIRB-001 cream were examined in 6-week clinical-dermatological application test in 25 subjects suffering from mild to moderate AD. With twice-daily application, SIRB-001 was very well tolerated and led to significant inhibition (p<0.001) in eczema area and severity index (EASI) with reduction of erythema, induration, excoriation and lichenification at 4 weeks and 6 weeks. Efficacious effect and tolerability of SIRB-001 cream was also evaluated in subjects with eczematous lesions in a mono-centric, open label study with twice daily application for 4 weeks. SIRB-001 cream demonstrated significant (p<0.001) decrease in eczema severity index (ESI), investigator's global assessment severity (IGAS) and was well-tolerated in human patients with a good safety profile. Inhibition of cytokines contributing to pathogenesis of AD; IL-8, IL-17A, TARC in serum samples was observed. It can be concluded that SIRB-001 is a highly effective new treatment with favorable safety profile for management of AD.
Keynote Forum
Michael R Migden
Associate Professor, Departments of Dermatology, Division of Internal Medicine, and Head and Neck Surgery, Division of Surgery, at the University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Keynote: Investigator-assessed efficacy and safety of sonidegib 200 mg QD in patients with locally advanced basal cell carcinoma: Results of the BOLT 30-month analysis
Time : 09:00 to 09:30

Biography:
Michael R. Migden, MD is a distinguished US-based dermatologist and Associate Professor, Departments of Dermatology, Division of Internal Medicine, and Head and Neck Surgery, Division of Surgery, at the University of Texas MD Anderson Cancer Center, Houston, TX, USA. At MD Anderson, he is the program director of the ACGME Fellowship: Micrographic Surgery and Dermatologic Oncology. He also serves as faculty for the Department of Ophthalmic Plastic & Reconstructive Surgery. He has served as a principal investigator for studies on the smoothened inhibitors sonidegib, vismodegib, and taladegib, and on immune therapy trials in non-melanoma skin cancer. Dr Migden has published numerous primary and expert review articles on basal cell carcinoma.
Abstract:
Statement of the Problem: The 200-mg dose of sonidegib, a selective smoothened inhibitor that blocks hedgehog pathway signaling, was approved in Europe and the United States for the treatment of patients with locally advanced basal cell carcinoma (laBCC) who are not amenable to curative surgery/radiation. Approvals were based on results from the pivotal BOLT study (NCT01327053). Investigator-assessed efficacy and safety data of sonidegib 200 mg QD from the 30-month analysis are reported here.
Methodology & Theoretical Questions: Patients with laBCC were randomized to receive sonidegib 200 or 800 mg daily; here we discuss the 200-mg dose. Investigators evaluated objective response rate (ORR); complete response [CR]+partial response [PR]), duration of response (DOR), and progression-free survival (PFS) per modified response evaluation criteria in solid tumors (mRECIST; laBCC) and RECIST v1.1 (mBCC); overall survival (OS) was also assessed.
Findings: In patients with laBCC who received sonidegib 200 mg (n=66), the investigator-assessed ORR was 71%. Disease control rate was 91%; median DOR was 15.7 months, with 25/47 responders maintaining an objective response. The median PFS was 19.4 months and the median OS was not yet reached. One death was reported; it was not considered related to study treatment by investigators. The safety profile of sonidegib 200 mg was manageable; however, grade 3/4 adverse events (AEs; 43%), AEs requiring dose interruptions/reductions (43%), and/or discontinuations due to AEs (30%) were reported. Commonly reported AEs included muscle spasms (54%), alopecia (58%), and dysgeusia (60%).
Conclusion & Significance: In the BOLT 30-month analysis, sonidegib 200 mg QD provided sustained efficacy and long-term safety in patients with laBCC. Notably, these data were investigator-assessed, which are typically higher than data that are centrally reviewed. These data support the use of sonidegib 200 mg in difficult-to-treat patients with laBCC according to local treatment guidelines.
Image
Figure: Efficacy of sonidegib 200 mg QD by investigator review in the BOLT 30-month analysis. CI=confidence interval; DCR, disease control rate; DOR=duration of response; NR=not reached; ORR=objective response rate; OS=overall survival; PFS=progression-free survival; aProgressive disease or death; bKaplan-Meier estimate
Recent Publications
1. Chen L, Silapunt S, Migden M R (2016) sonidegib for the treatment of advanced basal cell carcinoma: A comprehensive review of sonidegib and the BOLT trial with 12-month update. Future Oncol. 12(18): 2095-105.
2. Dummer R, Guminski A, Migden M R (2016) The 12-month analysis from Basal cell carcinoma outcomes with LDE225 treatment (BOLT): A phase II, randomized, double-blind study of sonidegib in patients with advanced basal cell carcinoma. J. Am. Acad. Dermatol. 75(1): 113-125.
3. Migden M R, Guminski A, Gutzmer R (2015) Treatment with two different doses of sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): A multicentre, randomized, double-blind phase 2 trial. Lancet Oncol. 16(6): 716-28.
