
Mel Ziman
Professor Edith Cowan University Australia
Title: Blood based biomarkers for diagnosis, prognosis and monitoring of patients with melanoma.
Biography
Biography: Mel Ziman
Abstract
Current methods of melanoma diagnosis and prognosis are at times problematic and limited to observation of tumour tissue by histology or imaging. The analysis of blood based, tumour specific products including autoantibodies, circulating tumour DNA (ctDNA) and circulating tumour cells (CTCs), now provides early rapid, accurate and quantitative measurements of tumour presence and /or burden. In our studies, we utilised protein arrays, mutation-specific droplet digital PCR and microfluidic devices to measure autoantibodies, mutant tumour DNA (ctDNA) and circulating tumour cells (CTCs), respectively, in patients with very early to advanced stage metastatic melanoma. Autoantibodies were detected in very early stage patients (n=150) at significantly higher concentrations than those in healthy controls (n=150). A diagnostic combination of 10 autoantibodies has been identified that can be utilised as an accompaniment to current clinical measures.
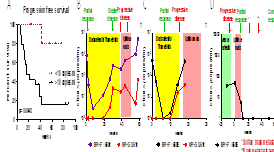
For metastatic melanoma we utilised ctDNA and CTCs to detect and monitor tumour burden during treatment of patients with targeted therapies (n=47) and/or immunotherapies (n=48). CTCs and/or ctDNA were detected in 70% to 80% of samples prior to treatment. Levels of ctDNA and CTCs decreased in response to therapies, prior to, or concurrently with radiographic response. Moreover, patients with no, or low, levels of ctDNA and CTCs at baseline had significantly longer PFS. In addition, CTC subtypes, including those positive for PDL1, predicted response. In conclusion, our studies demonstrate the utility of blood based liquid biopsies to assist with diagnosis, prognosis and monitoring of melanoma patients.
Recent Publications
1. E Gray, A Reid, S Bowyer, L Calapre, K Siew, R Pearce, L Cowell, M H Frank, M Millward, M Ziman (2015) Circulating melanoma cell subpopulations: Their heterogeneity and differential responses to treatment. J. Invest. Dermatol 135(8):2040-2048.
2. E Gray, H Rizos, A Reid, S Boyd, M Pereira, J Lo, V Tembe, J Freeman, J Lee, R Scolyer, K Siew, C Lomma, A Cooper, M Khattak, T Meniawy, G Long, M Carlino, M Millward, M Ziman (2015) Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma, Oncotarget 6(39):42008-18.
3. D Klinac, E Gray, J Freeman, A Reid, S Bowyer, M Millward, M Ziman (2014) Monitoring changes in circulating tumor cells as a prognostic indicator of overall survival and treatment response in patients with metastatic melanoma. BMC Cancer 14:423.
4. A Reid, M Millward, R Pearce, M Lee, M H Frank, P Heenan, A Ireland, L Monshizadeh, T Rai, S Medic, M Ziman (2013) Markers of circulating tumor cells in the peripheral blood of melanoma patients correlates with disease recurrence and progression. BJD 168: 85-92.
5. J Freeman, E Gray, M Millward, R Pearce, M Ziman (2012). Evaluation of a multi-marker immunomagnetic enrichment assay for the quantification of circulating melanoma cells. J. Transl. Med. 10: 192.

